By Jon Plant, DVM, DACVD
Atopica® (cyclosporine) and Apoquel® (oclacitinib) are separate and distinct medications. Both are used for controlling the signs and symptoms of atopic dermatitis (“allergies”) in dogs. They are two of the most effective allergy treatments for dogs. Let’s see how they compare.
Availability of Atopica and Apoquel
Atopica was FDA approved more than 10 years ago and is widely available as a prescription drug through veterinarians.
Apoquel was launched in January 2014, but the demand quickly exceeded the manufacturing capacity of the manufacturer, Zoetis. This has led to very limited availability and backorders. Most veterinary clinics are unable to order any Apoquel at all. Zoetis anticipates that production will be able to keep up with the demand in the spring of 2015.
Cost of Atopica and Apoquel
Atopica comes in four sizes: 10, 25, 50 and 100 mg capsules. It is dosed based on your dog’s body weight. Each size is priced differently and larger dogs may require more than one capsule. Depending upon your dog’s weight, the initial cost may range from $1.50-$10 per day. Significant rebate programs are often available when purchases are made through veterinarians (as much as 50% off ) but not through online pharmacies. The cost often goes down over time if the dose is able to be reduced.
Apoquel comes in three sizes: 3.6, 5.4, and 16 mg tablets. Dogs less than 90 pounds need only take 0.5 or 1.0 tablet per day, long term. Big dogs will require 1.5 or 2.0 tablets per day. A novel feature of Apoquel is that all three tablets are priced the same. There isn’t much information on the retail pricing of Apoquel available, but it is likely to be around $1.50-$2.00 per tablet in most veterinary hospitals that have it in stock.
Dosing of Atopica and Apoquel
Atopica comes as capsules, which are fairly large in the 100 mg size. Pet owners often find the larger size difficult to administer. The initial dose is 5 mg per kg body weight. In most cases, it is given once daily for the first month. If your dog responds well, the dose can often be reduced to every 48 hours or even twice weekly. It is then given long-term, or at least during the seasons that your dog itches from allergies.
Apoquel comes as scored tablets, which are fairly small and easy to administer. There is a narrow dose range of 0.4-0.6 mg per kg of body weight. Your veterinarian will sometimes need to use half of two different sizes to get the proper dose. For up to 14 days, Apoquel is administered twice daily. In cases of chronic itch in dogs, it is given once daily, long term. Apoquel has a short half-life, meaning that it doesn’t persist for long in the blood stream. Missing even one dose may result in a return of the itching behavior. Establishing a daily routine or setting an iPhone reminder is important. Check out the Itchology app on Facebook, which has a built in medication reminder.
Speed of Atopica and Apoquel in reducing itch
Atopica does not usually achieve its maximum effect on itching until after daily dosing for four weeks.
Apoquel reduces itching quickly, often within one day. There is a major reduction in itching within 7 days in most dogs. In a head-to-head study, Apoquel reduced the itch level more than Atopica during the first 14 days. There is often a slight increase in itch level when Apoquel dosing is switched from twice daily to once daily, usually at 14 days of therapy.
Side effects of Atopica and Apoquel
Both Atopica and Apoquel affect the immune system. An allergy is, after all, an overactive immune system. Atopica is considered immunosuppressive, effecting T-cells. Apoquel is considered immunomodulatory, blocking transmission of the itch sensation, among other activities. Both medications have the potential to increase the risk of dogs getting infections. In reality, this is uncommon at recommended doses. In clinical trials, skin infections (pyoderma) do occur, but dogs with allergies often get skin infections whether they are taking one of these medications or not.
Atopica is associated with vomiting and diarrhea more often than Apoquel. In a review study compiling results of 672 dogs treated with Atopica, vomiting occurred in 25% and diarrhea or soft stools in 15% of dogs. Usually, veterinarians and pet owners can overcome this, with a slight modification of dosing.
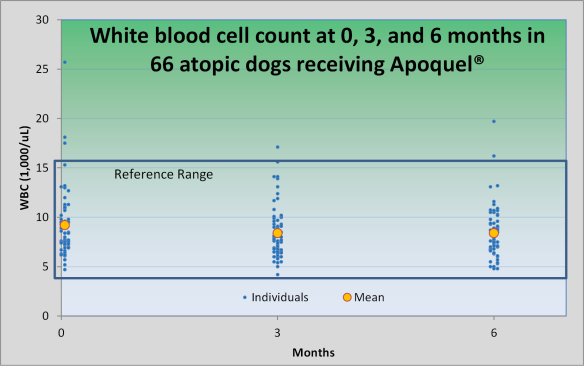
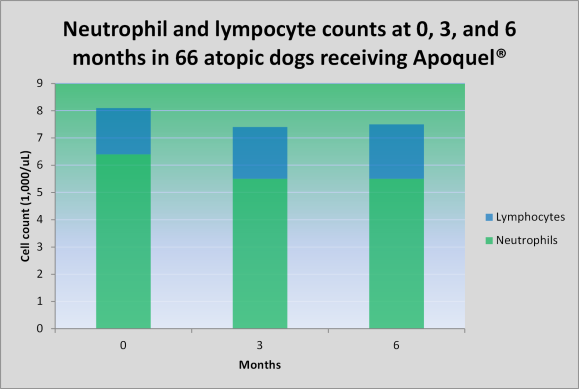
Apoquel is uncommonly associated with vomiting or soft stools (1-2% of dogs). In most studies, these occur with a similar frequency in placebo-treated dogs and those treated with Apoquel. Because Apoquel is still relatively new, it is prudent to monitor our patients rather closely. I recommend an examination, complete blood panel and urinalysis at 0, 3, and 6 months, then every 6 months while taking Apoquel, for now.







 e leaves veterinarians with the traditional methods of controlling atopic dermatitis (“allergies”). The first step is to make the correct diagnosis by using a validated checklist and ruling out alternative diagnoses (see my short video on
e leaves veterinarians with the traditional methods of controlling atopic dermatitis (“allergies”). The first step is to make the correct diagnosis by using a validated checklist and ruling out alternative diagnoses (see my short video on 